An effortless and comprehensive approach to burden of illness reviews
Blog - Cost-utility models in non-small-cell lung cancer
Published: 06-12-2016
Cost-utility models are at the core of many health technology assessments and can be an essential step to getting market access for new products. The National Institute for Health and Care Excellence (NICE) requires manufacturers to have conducted a literature review to identify existing cost-utility models as part of the development process for their own model. Yet finding these models takes time.
We used the heoro.com database to search rapidly for cost-utility models in non-small-cell cancer (NSCLC) and to summarise the results in an evidence map. The image below shows how we set up the dashboard for the search.
We found 70 abstracts published since 1960 that reported on cost-utility models for interventions in NSCLC. We exported the results into a spreadsheet and categorised each abstract according to jurisdiction, disease stage, intervention type and year of publication to create the evidence maps.The whole process took approximately 3 hours.
Of the 70 abstracts, 18 were set in the United States, 12 in the United Kingdom, 7 in China, 5 in the Netherlands and 4 each in France and Canada. A further 7 were international studies or literature reviews.
The 70 abstracts modelled a wide range of interventions. Ten focused on strategies for screening, staging the disease or surveillance after initial therapy, and three modelled the cost-utility of palliative or supportive care strategies, including treatments for bone metastases. Not surprisingly, the majority of papers modelled the cost-utility of treatments for NSCLC, with 30 including targeted treatments (mainly erlotinib and gefitinib), 22 including chemotherapy, including taxanes and platinum compounds, 7 radiotherapy and 3 surgery.
The geographical breakdown of abstracts by intervention type is shown in the table below. Please note that the table includes some double-counting, as some models compared different treatment types so that abstract will be recorded as modelling two different treatment types.
Map of cost-utility studies by intervention type and jurisdiction
| Total abstracts | Chemotherapy | Targeted therapy | Surgery | Radiotherapy | Screening/ surveillance/ staging | Palliative care | Any/all | |
| Belgium | 1 | 1 | ||||||
| Brazil | 1 | 1 | ||||||
| Canada | 4 | 1 | 1 | 2 | ||||
| China | 7 | 2 | 6 | |||||
| France | 4 | 3 | 3 | |||||
| Germany | 1 | 1 | ||||||
| International | 7 | 1 | 1 | 3 | 1 | 1 | ||
| Japan | 3 | 1 | 1 | 1 | ||||
| Netherlands | 4 | 3 | 1 | |||||
| Singapore | 1 | 1 | ||||||
| Spain | 2 | 1 | 1 | |||||
| Switzerland | 2 | 1 | 1 | |||||
| Thailand | 2 | 2 | 1 | |||||
| UK | 12 | 4 | 6 | 1 | 1 | |||
| Unclear | 1 | 1 | ||||||
| US | 18 | 3 | 9 | 3 | 2 | 3 | 1 | |
| Total | 70 | 22 | 30 | 3 | 7 | 10 | 3 | 2 |
Most of the studies modelled the cost-utility of interventions for advanced disease (28 abstracts), with a further 10 focusing on metastatic disease or the combined advanced or metastatic population. Nine abstracts looked at interventions for early disease and 23 abstracts did not specify or included any stage. The details are shown in the table below.
Map of cost-utility studies by disease stage and jurisdiction
| Early | Advanced | Metastatic | Advanced or metastatic | All or any stage | Total | |
| Belgium | 1 | 1 | ||||
| Brazil | 1 | 1 | ||||
| Canada | 1 | 3 | 4 | |||
| China | 5 | 1 | 1 | 7 | ||
| France | 2 | 2 | 4 | |||
| Germany | 1 | 1 | ||||
| International | 1 | 1 | 1 | 4 | 7 | |
| Japan | 3 | 3 | ||||
| Netherlands | 2 | 1 | 1 | 4 | ||
| Singapore | 1 | 1 | ||||
| Spain | 2 | 2 | ||||
| Switzerland | 2 | 2 | ||||
| Thailand | 2 | 2 | ||||
| UK | 2 | 1 | 5 | 4 | 12 | |
| Unclear | 1 | 1 | ||||
| US | 5 | 6 | 2 | 5 | 18 | |
| Total | 9 | 28 | 4 | 6 | 23 | 70 |
Although our search went back to 1960, the earliest publication date on the models we found was 1995, and the majority of papers were published since 2010, reflecting the launch of the new targeted therapies. The chart below shows the trend in cost-utility models over time. Again, where a model compares a tartgeted therapy with chemotherapy, it has been categorised under both treatment types, so the numbers add up to more than the 70 abstracts we found.
Trends in cost-utility modelling over time
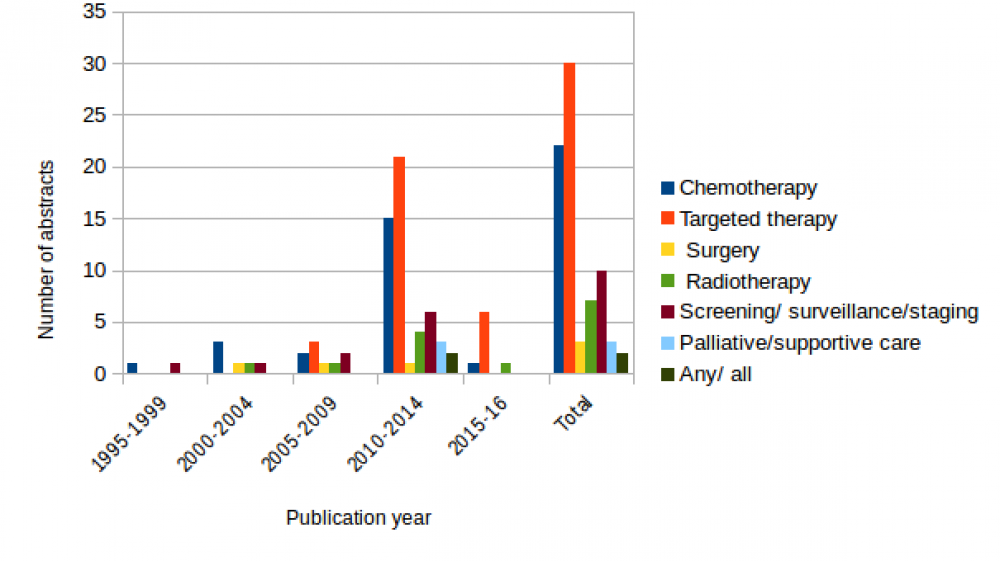
Our evidence map shows the impact of the new targeted therapies on cost-utility models in NSCLC. These included erlotinib, gefitinib, crizotinib, icotinib, bevacizumab, necitumumab and cetuximab. Nine studies modelled the cost-utility of genetic screening for EGFR or ALK mutation status and then offering targeted therapy to the appropriate subgroups only. It is not surprising that the UK, with market access driven by the stringent requirements of NICE, was the setting for 12 models. However, the US and China came first and third in the list, suggesting that the need to demonstrate cost-effectiveness now extends way beyond jurisdictions with a strong, established HTA barrier to market access.