An effortless and comprehensive approach to burden of illness reviews
Blog - Cost-utility models in multiple sclerosis
Published: 28-12-2016
When developing an economic model, it is important to review existing models for the disease and interventions of interest, to determine the best modelling approach, data sources and assumptions to use. However, finding this information can be time-consuming. We have used the heoro.com database to speed up this process. In this blog, we describe the evidence map we've generated of cost-utility models in multiple sclerosis (MS).
We searched the heoro.com database for the disease Multiple sclerosis, study type Economic models and selected cost-utility models from the list of options, as shown below.
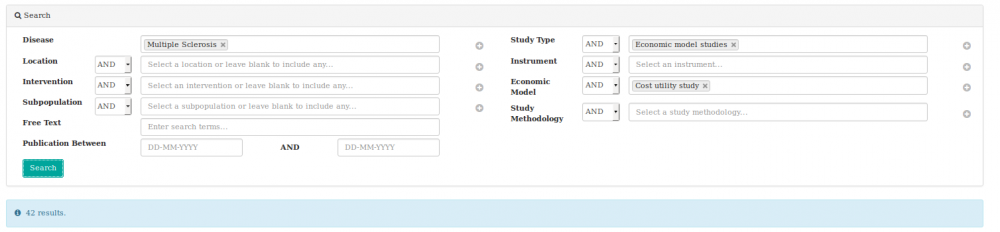
We identified 42 relevant abstracts. Of these, 14 were on relapsing-remitting MS (RRMS), six were on primary or secondary progressive MS and 22 were on any type of MS or did not specify. Most models assessed the cost-utility of interferons, or compared these with newer treatments. Fifteen abstracts modelled the use of interferon beta-1b, 11 interferon beta-1a and 7 evaluated any type of interferon-beta. Fourteen abstracts modelled the cost-utility of glatiramer acetate, four natalizumab and three each fingolimod, mitoxantrone and cannabinoids (please note that totals add up to more than 42 abstracts as each intervention has been counted, rather than each model).
The jurisdiction of fourteen models was the United Kingdom, for ten it was the United States, four were set in Sweden, three in Spain, two in Germany (including one abstract that modelled cost-utilities for both Spain and Germany) and 7 were international literature reviews or set in multiple countries.
Twenty-one of the models used a societal perspective and nine a healthcare payer perspective, with 10 abstracts not specifying this. Of the 42 abstracts, 23 were published between 2010 and 2016 and none were published before 1997. The evidence map is shown below. It took approximately three hours to generate.
Number of abstracts identified for each type of MS, intervention and jurisdiction
| Any IFN-beta | Interferon beta-1a | Interferon beta-1b | Glatiramer | Fingolimod | Natalizumab | Mitoxantrone | Dimethyl fumarate | Teriflunomide | Botulinum toxin | Cannabinoids | |
| Type of MS | |||||||||||
| RRMS | 2 | 8 | 7 | 9 | 3 | 3 | 2 | 1 | |||
| Progressive MS | 1 | 4 | 1 | 1 | 1 | ||||||
| Any or unspecified | 2 | 2 | 4 | 4 | 1 | 2 | 1 | 2 | |||
| Jurisdiction | |||||||||||
| France | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| Germany | 1 | 1 | 1 | 1 | |||||||
| International | 1 | 1 | 1 | 1 | |||||||
| Italy | 1 | ||||||||||
| Serbia | 1 | 1 | 1 | ||||||||
| Spain | 2 | 2 | 2 | 1 | |||||||
| Sweden | 1 | 2 | 1 | ||||||||
| UK | 4 | 1 | 2 | 3 | 1 | 1 | 1 | 1 | 1 | 2 | |
| US | 1 | 4 | 6 | 5 | 1 | 1 | 1 | ||||
| Totals | 7 | 11 | 15 | 14 | 3 | 4 | 3 | 2 | 1 | 1 | 3 |
Number of abstracts identified for each modelling perspective, by jurisdiction
| Country | Healthcare perspective | Societal perspective | Unspecified perspective |
| France | 1 | ||
| Germany | 1 | 1 | |
| International | 1 | 5 | |
| Italy | 1 | ||
| Serbia | 1 | ||
| Spain | 1 | 2 | |
| Sweden | 4 | ||
| UK | 7 | 3 | 3 |
| US | 1 | 7 | 2 |
| Total | 9 | 21 | 10 |
The evidence map shows that the majority of cost-utility models published for treatments of multiple sclerosis assessed interferon beta formulations, or compared these treatments against newer products, and were generally relevant to the UK or US jurisdiction. The substantial impact of the disease on social and occupational functioning was reflected in the high proportion of models that were conducted from a societal perspective.